Objectives:
Toxoplasma gondii (T. gondii) is an important zoonotic parasite for human and animal health. Humans and animals can become infected with T. gondii by ingesting either tissue cysts from raw or undercooked meat, or sporulated oocysts from environmental sources. T. gondii oocysts are released in the faeces of cats and other felids, which are the parasite's definitive hosts, leading to environmental contamination. The parasite therefore has several transmission routes, although meat from infected animals appears to be a major source of human infection in Europe. The research interests of the Toxoplasma group thus focus on the presence of T.gondii in production animals (particularly those intended for human consumption), domestic animals and wild animals, mainly in Europe but also worldwide. Host-pathogen interactions and the contribution of human sources of infection are also major concerns, in line with the OneHealth concept.
Our research has several objectives:
I- To monitor the epidemiology and circulation of T. gondii in farm, domestic and wild animals using standardised serological techniques in order to increase knowledge of the seroprevalence of T. gondii in animals in European countries and throughout the world.
Modified Agglutination Test using T.gondii antigens (kindly provided by CNR Toxoplasma, Reims, France) and pig serums ©F.Damek, S. Thoumire, L Grbavac, D Le Roux and R Blaga, BIPAR JRU
II- Establish an assessment of the risk factors for infection of different livestock species and identify the sources of T. gondii contamination of humans, so that more appropriate preventive measures can then be proposed.
Human Foreskin Fibroblast (HFF) cells and infected with T.gondii tachyzoites (Mic1-3KO-GFP strain, kindly provided by Vitamfero) ©D Le Roux and R Blaga, BIPAR JRU
III- To characterise the factors involved in the sexual cycle of T. gondii in its definitive host (the cat) and the mucosal immune response, with the ultimate aim of identifying new targets for control by vaccination in line with the One Health concept, linking animal health, the environment and human health.
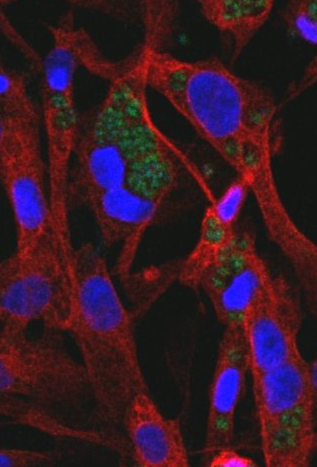
Feline endothelial cells stained with DAPI (nucleus, blue), a-tubulin (red) and infected with T.gondii tachyzoites (PruYFP strain, green, kindly given by D. Soldati, switzerland) ©J. Rouzet and D Le Roux, BIPAR JRU
We regularly welcome students on short-term placements (BTS, DUT, L3, M1 and M2), as well as visiting French and international researchers.
Major results:
I- Among the various species intended for human consumption, sheep appear to present a high risk of human infection. The results of this study suggest that lamb meat poses a risk to consumers if it is undercooked or poorly cooked.
Anatomical distribution of Toxoplasma gondii in naturally and experimentally infected lambs. 2022, Thomas et al., Parasite.
II- Chickens are often used as sentinels of environmental contamination by T. gondii, especially if they are free-range. The aim of this study was to investigate the abundance and specific distribution of T. gondii cysts in the brains of experimentally infected chickens. The number of T. gondii cysts found in chicken brains appears to be considerably lower than in rodent studies.
Burden and regional distribution of Toxoplasma gondii cysts in the brain of COBB 600 broiler chicken following infection with 76K strain. 2021, Beck et al., Veterinary Parasitology
III- T. gondii can cause reproductive defects (e.g. abortions). The aim of this study was to examine the effect of experimental T. gondii infection on semen quality in rams. In conclusion, prepubertal toxoplasmosis in rams adversely affected sperm quality in mature animals, and administration of sulphadimidine failed to alter this situation.
Effect of Toxoplasma gondii on Ram Sperm Quality After Experimental Infection. 2020, Fais et al., Pathogens
IV- Vaccination of the feline host against T. gondii is an interesting strategy for interrupting the life cycle of the parasite and subsequently limiting contamination of the environment and intermediate hosts. In this study, a new attenuated live vaccine strain of T. gondii, which had previously been shown to be effective in murine and ovine models, was tested in cats. The results show that this candidate vaccine is immunogenic in the feline host, well tolerated and safe, but does not confer protection against oocyst excretion following natural infection with the parasite.
Evaluation of immunogenicity and protection of the Mic1-3 knockout Toxoplasma gondii live attenuated strain in the feline host. 2020, Le Roux et al., Vaccine
V- In France, consumption of beef and sheep meat appears to be a risk factor for T. gondii infection in pregnant women, but little is known about the prevalence of the parasite in beef. The main aim of this study was to estimate the seroprevalence of T. gondii infection in beef consumed in France. Seroprevalence estimates and molecular investigations indicated that there is a limited risk of human infection by T. gondii, which must be correlated with the dietary habit of consuming raw or undercooked beef.
Toxoplasma gondii in beef consumed in France: regional variation in seroprevalence and parasite isolation. 2019, Blaga et al., Parasite
Current projects:
- ToxSauQMRA: Pork is the most widely consumed meat in France, with dry sausages well represented. The risk of transmission through consumption of processed pork products is largely unknown, mainly because processing will affect viability, but may not fully inactivate all T. gondii parasites. The data from this project will form the basis of a quantitative microbial risk assessment (QMRA) model for meat products specific to France, aimed at estimating the main sources of human T. gondii infection from consumption of these specialities.
- OH EJP Toxosources : This European consortium, in which our group is involved, is addressing the following research question: what are the relative contributions of the different sources of human infection with T. gondii - using several multidisciplinary approaches and new and improved methods to produce the most robust estimates possible that can inform risk management and policy makers. The QMRA model is one of the main outputs of the joint European One Health Toxosources project. In this model, we aim to estimate the relative contribution of the different sources of human infection by T. gondii.
- AMI GamApi : Current methods of controlling T. gondii and E. acervulina, two parasites with a similar life cycle, on livestock farms do not target sexual reproduction, leading to the production of the new generation of infectious forms of these two parasites: oocysts. This project therefore focuses on the sexual cycle of these two model coccidia, given their major economic and societal impact, in order to identify new therapeutic and/or prophylactic targets, using new in vitro and ex-vivo study models.
- Study of the seroprevalence of T. gondii in Croatian pigs and evaluation of risk factors linked to the biosecurity category of livestock farms, in collaboration with the Faculty of Veterinary Medicine at the University of Zagreb, Croatia.
- Tropism of Toxoplasma gondii in the tissues of experimentally infected pigs in collaboration with the Institute of Veterinary Research in Brno, Czech Republic.
- Detection of Toxoplasma gondii-specific antibodies in pigs using a commercial ELISA based on oral fluids: advantages and limitations
Collaborations:
- Consortium ToxoSource (European Joint Project): a consortium of institutions from several European countries: Denmark, the Netherlands, Italy, Germany, Spain, France, Poland, Portugal, Norway, the United Kingdom, the Czech Republic, Sweden, Lithuania, Ireland, Greece and Romania.
- Faculty of Veterinary Medicine, University of Veterinary Sciences, Brno, Czech Republic
- Department of Parasitology and Parasitic Diseases with clinics, Faculty of Veterinary Medicine, University of Zagreb, Croatia
- Toxoplasma National Reference Centre and Biological Resource Centre, Reims France
- Institute of Parasitology, Vetsuisse-Faculty, University of Bern, Länggassstrasse 122, CH-3012 Bern, Switzerland
Members of the group:
Manager: Radu BLAGA
- Researchers / Assistant Professor
- PhD students